Réglementation Pharmaceutique Mondiale
L’une des premières réglementations pharmaceutiques a été mise en œuvre par la Turquie en 2009. Depuis, de nombreux pays ont suivi et mis en œuvre diverses réglementations pour la sérialisation et l’agrégation des produits pharmaceutiques. Chez Laetus, nous avons soutenu nos clients dès le début, en mettant en œuvre des solutions innovantes pour assurer leur conformité.
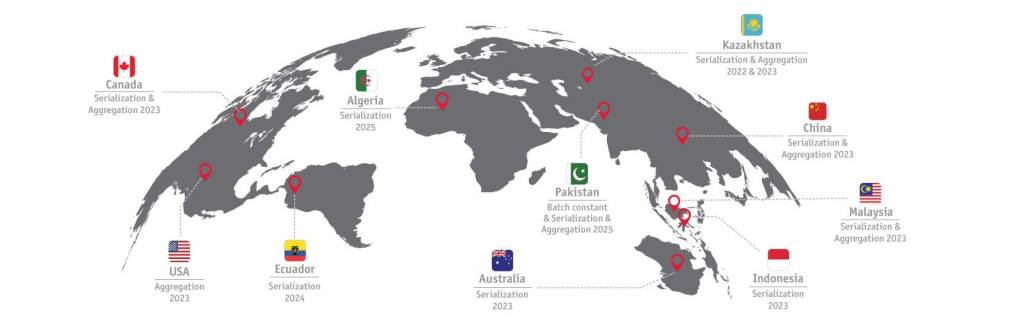
Le graphique ci-dessus met en évidence les exigences réglementaires récentes et à venir.
La section suivante énumère tous les pays qui ont mis en place des réglementations pour la sérialisation et l’agrégation (un PDF peut être téléchargé au bas de cette page) :
Algeria 
Ministry of Health, Population and Hospital Reform (MSPRH)
Serialization
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix |
| Deadline | Planned for 2025 |
Click here to go to local authority.
Argentina 
Ministry of Health of the Argentine Republic (ANMAT)
Serialization
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix |
| Deadline | 01 January 2023 |
Click here to go to local authority.
Australia 
Department of Health and Aged Care
Serialization
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix |
| Deadline | 01 January 2023 |
Click here to go to local authority.
Bahrain 
National Health Regulatory Authority (NHRA)
Serialization
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix |
| Deadline | 31 December 2019 |
Aggregation
| Data carrier | GS1 128 |
| Deadline | 01 September 2021 |
Click here to go to local authority.
Brazil 
Brazilian Health Regulatory Authority (ANVISA) RDC 157 / 2017
Serialization
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(713) – NHRN AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix |
| Deadline | 01 April 2022 |
Aggregation
| Data elements | AI(00) – SSCC |
| Data carrier | GS1 128 |
| Deadline | 01 April 2022 |
Click here to go to local authority.
Canada 
Canadian Institute for Safe Medication Practices (ISMP)
Batch constant
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN |
| Data carrier | GS1 DataMatrix GS1 128 |
| Deadline | 31 December 2023 |
Serialization
| Data elements | optional AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix GS1 128 |
| Deadline | 31 December 2023 |
Click here to go to local authority.
Chile 
Ministerio de Salud, Sub Secretaria de Salud Publica – Division Juridica
Batch constant
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN |
| Data carrier | GS1 DataMatrix GS1 128 |
| Deadline | 2013 |
Click here to go to local authority.
China 
National Medicinal Products Administration (NMPA)
Serialization
| Data elements | Proprietary Chinese eCode or AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | GS1 128 |
| Deadline | June 2023 |
Aggregation
| Data elements | Proprietary Chinese eCode or AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | GS1 128 |
| Deadline | June 2023 |
Click here to go to local authority.
Columbia 
INVIMA Ministry of Health and Social Protection
Serialization
| Data elements | AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | RFID |
| Deadline | 01 April 2018 |
Click here to go to local authority.
Ecuador 
National Agency for Health Regulatory Control and Surveillance (ARCSA)
Serialization
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix GS1 128 |
| Deadline | 26 May 2024 |
Click here to go to local authority.
Egypt 
Egyptian Ministry of Health
Serialization
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix GS1 128 |
| Deadline | 31 December 2018 |
Click here to go to local authority.
Europe 
EU Commission
Directive 2011/62/EU
Serialization
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(713) – NHRN if applicable AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix DataMatrix |
| Deadline | 09 February 2019 |
Click here to go to local authority.
India 
Ministry of Health and Family Welfare of India
Serialization
| Primary | |
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix GS1 Databar |
| Deadline | 01 April 2015 |
| Secondary | |
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix GS1 128 GS1 Databar |
| Deadline | 01 October 2013 |
| Tertiary | |
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(21) – Serial Number AI(00) – SSCC |
| Data carrier | GS1 DataMatrix GS1 128 |
| Deadline | 01 October 2011 |
Aggregation
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(00) – SSCC |
| Data carrier | GS1 128 |
| Deadline | 01 July 2019 |
Click here to go to local authority.
Indonesia 
National Agency for Pharmaceuticals and Food Control of the Republic of Indonesia
Serialization
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix |
| Deadline | 01 January 2023 |
Click here to go to local authority.
Iran 
Ministry of Health and Medical Education
Serialization
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN UID |
| Data carrier | GS1 DataMatrix |
| Deadline | 01 January 2015 |
Click here to go to local authority.
Japan 
Ministry of Health, Labour and Welfare
Serialization
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | GS1 DataBar |
| Deadline | 01 July 2015 |
Aggregation
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(21) – Serial Number AI(30) – Quamtity |
| Data carrier | GS1 128 |
| Deadline | 01 April 2021 |
Click here to go to local authority.
Jordan 
Jordanian Food and Drug Administration (JFDA)
Batch constant
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN |
| Data carrier | GS1 DataMatrix |
| Deadline | 01 July 2018 |
Serialization
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix |
| Deadline | 01 January 2020 |
Click here to go to local authority.
Kazachstan 
Ministry of Finance / Health
Serialization
| Data elements | AI(01) – GTIN AI(21) – Serial Number AI(91) – Crypto Key AI(92) – Crypto Code |
| Data carrier | GS1 DataMatrix |
| Deadline | 01 October 2011 (20%) 01 January 2023 (60%) 01 April 2023 (80%) 01 July 2023 (100%) |
Aggregation
| Data elements | AI(00) – SSCC |
| Data carrier | GS1 128 |
| Deadline | 01 October 2011 (20%) 01 January 2023 (60%) 01 April 2023 (80%) 01 July 2023 (100%) |
Click here to go to local authority.
Lebanon 
Ministry of Public Health
Batch constant
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN |
| Data carrier | GS1 DataMatrix |
| Deadline | 31 December 2019 |
Click here to go to local authority.
Malaysia 
Malaysian Ministry of Health
Serialization
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix |
| Deadline | 01 January 2023 |
Aggregation
| Data elements | AI(00) – SSCC |
| Data carrier | GS1 128 |
| Deadline | 01 January 2023 |
Click here to go to local authority.
Norway 
The Norwegian Medicines Agency
Serialization
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix |
| Deadline | 09 February 2019 |
Click here to go to local authority.
Oman 
Directorate General of Medical Supplies MoH
Batch constant
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN |
| Data carrier | GS1 DataMatrix |
| Deadline | 31 December 2017 |
Serialization
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix |
| Deadline | 01 March 2019 |
Click here to go to local authority.
Pakistan 
Pakistan Drug Regulatory Authority (DRAP)
Batch constant
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN |
| Data carrier | GS1 DataMatrix |
| Deadline | 01 August 2025 |
Serialization
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix |
| Deadline | 01 August 2025 |
Aggregation
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(21) – Serial Number AI(240) – Product |
| Data carrier | GS1 DataMatrix |
| Deadline | 15 September 2025 |
Click here to go to local authority.
Philippines 
Food and Drug Administration Philippines
Batch constant
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN |
| Data carrier | GS1 DataMatrix GS1 128 |
| Deadline | 30 June 2016 |
Click here to go to local authority.
Quatar 
Hamad Medical Corporation (HMC)
Batch constant
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN |
| Data carrier | GS1 DataMatrix GS1 128 |
| Deadline | 01 January 2018 |
Serialization
| Data elements | AI(21) – Serial Number, if applicable |
| Data carrier | GS1 DataMatrix GS1 128 |
| Deadline | 01 January 2018 |
Click here to go to local authority.
Russia 
Ministry of Health of the Russian Federation
Serialization
| Data elements | AI(10) – Batch/Lot AI(21) – Serial Number AI(91) – Crypto Key AI(92) – Crypto Code |
| Data carrier | GS1 DataMatrix |
| Deadline | 01 July 2020 |
Aggregation
| Data elements | AI(00) – SSCC or AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix GS1 128 |
| Deadline | 01 July 2020 |
Click here to go to local authority.
Saudi Arabia 
Saudi Food and Drug Administration (SFDA)
Batch constant
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN |
| Data carrier | GS1 DataMatrix |
| Deadline | 21 March 2015 |
Serialization
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix |
| Deadline | 12 March 2017 |
Aggregation
| Data elements | AI(00) – SSCC |
| Data carrier | GS1 128 |
| Deadline | 31 March 2020 |
Click here to go to local authority.
South Africa 
Ministry of Health of the Republic of South Africa (NDOH)
Serialization
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix GS1 QR |
| Deadline | 01 June 2022 |
Click here to go to local authority.
South Korea 
Ministry for Food and Drug Safety
Batch constant
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN |
| Data carrier | GS1 DataMatrix GS1 128 |
| Deadline | 01 January 2013 |
Serialization
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix GS1 128 RFID |
| Deadline | 01 January 2016 |
Click here to go to local authority.
Switzerland 
Swiss Federal Office of Public Health (FOPH)
Serialization
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTINoptional AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix |
| Deadline | 2018 |
Click here to go to local authority.
Tunesia 
Ministry of Public Health
Batch constant
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN |
| Data carrier | GS1 DataMatrix |
| Deadline | 01 January 2019 |
Click here to go to local authority.
Turkey 
Republic of Turkey Ministry of Health – Turkish Medicines and Medical Devices Agency (TİTCK)
Serialization
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix |
| Deadline | 01 June 2010 |
Aggregation
| Data elements | AI(00) – SSCC |
| Data carrier | GS1 128 |
| Deadline | 01 June 2012 |
Click here to go to local authority.
Ukraine 
State Service of Ukraine on Medicines and Drug Control
Serialization
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix |
| Deadline | 2021 (not defined) |
Click here to go to local authority.
UAE 
Department of Health – Abu Dhabi
Batch constant
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN |
| Data carrier | GS1 DataMatrix |
| Deadline | 01 January 2017 |
Serialization
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix |
| Deadline | 01 January 2020 |
Click here to go to local authority.
USA 
U.S. Food and Drug Administration (FDA)
Serialization
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix GS1 128 |
| Deadline | 27 November 2017 |
Aggregation
| Data elements | AI(10) – Batch/Lot AI(17) – Expiration Date AI(01) – GTIN AI(21) – Serial Number |
| Data carrier | GS1 DataMatrix GS1 128 |
| Deadline | 27 November 2023 |
Click here to go to local authority.
Uzbekistan 
The State Tax Committee of the Republic of Uzbekistan, the Ministry of Finance and the Ministry of Health
Serialization
| Data elements | AI(01) – GTIN AI(21) – Serial Number AI(91) – Crypto Key AI(92) – Crypto Code |
| Data carrier | GS1 DataMatrix |
| Deadline | 01 September 2022 |
Click here to go to local authority.
Téléchargez notre PDF qui résume les informations et fournit des liens vers les différents règlements.
Veuillez nous fournir les informations suivantes pour télécharger notre aperçu complet des règlements.
Dernière mise à jour : janvier 2023 – toutes les données sont sans garantie.
