
PHARMA-CODE Series: #1 Intro to Laetus PHARMA-CODE
PHARMA-CODE is a trademark of Laetus. We specialize in serialization and quality control hardware and software. PHARMA-CODE in all upper-case and hyphenated is the correct way to identify this unique symbology, in accordance with the patent.
PHARMA-CODE is older than UPC
Packaging of drugs often contains numerous component parts: labels, medicine leaflets, and other drug usage and contraindication data. The inclusion of all the correct inserts and no incorrect inserts is essential to drug packaging compliance. Applying the same code to each element of drug packages is an effective way to ensuring that only correct data is packaged with a drug. PHARMA-CODE assures packaging accuracy, preventing mix-ups of pharmaceutical product and packaging. Unlike virtually any other barcode, printing the PHARMA-CODE barcode in a range of colors verifies that the package contains the appropriate color-coded insert. In this regard, PHARMA-CODE greatly enhances the accuracy of drug packaging; it is not better than the HIBC or GS1 UDI—it is altogether different. Many pharmaceutical manufacturers continue to use PHARMA-CODE to identify and control drugs because they have used them for many years.
Preventing intermixture for over 50 years
It matches and validates all of the component parts of a package to ensure that the right product, with all of its required documentation, goes into the right package. PHARMA-CODE is only used for internal tracking. HIBC and GS1 compliant barcodes can also do the above requirements, yet both of them are managed by organizations, manufacturers need to pass various steps to certify or purchase HIBC and GS1 code. Thus from a return on investment perspective, using PHARMA-CODE technology to generate code is faster and less expensive—in fact free.

Furthermore, a standard does not specify the allocation of PHARMA-CODE data, as with most other barcode types. Users allocate the encoded data according to a procedure based on their particular packaging requirements. In contrast, UDI compliant barcodes comply with a specification that covers everything from number allocation to data structure; data field prefixes, content and sizes, and check digits.
Another difference with PHARMA-CODE is that encoded information does not correspond to the barcode’s human-readable characters. Encoded data is in numerical form but encoded in a binary mechanism. When a camera reads the binary code, a PHARMA-CODE decoder needs to be integrated to translate the binary code to a numeric format. PHARMA-CODE does not use the start or stop characters. Scanning a PHARMA-CODE is done right to left. Scanning left to the right produces a different result in line with the binary code characteristics.
PHARMA-CODE prevents packaging mistakes
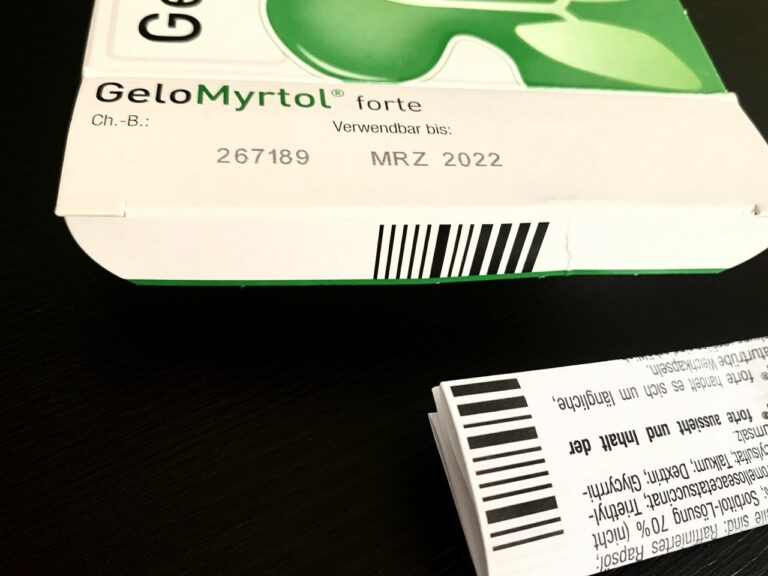
The key to understanding PHARMA-CODE is its closed-loop use case. UDI compliant barcodes serve an open-loop system that covers a wide range of environments. UDI barcodes travel from the manufacture and bulk packaging through distribution to receiving and inventory control, and retail pharmacy point of sale or bedside dosing in a health care facility. The closed-loop environment of a PHARMA-CODE is packaging – kitting the drug with all its constituent parts in the package. Errors at this stage jeopardize important federal compliance requirements.
Beyond the significant differences between PHARMA-CODE and open-loop symbology, there are important similarities. Barcode quality is paramount: whatever the use case, the barcode must work. This series will delve more deeply into the PHARMA-CODE standard, quality attributes, and verification.
The PHARMA-CODE is printed on an inside flap of the pharmaceutical package. It is not visible at point-of-sale.
Continue to part 2 of our PHARMA-CODE series.
*Co-authored with John Nachtrieb, CEO of Barcode Test.