UDI & Serialization of Medical Devices
The deadline for implementing traceability for medical devices is in the European Union is approaching. By the end of 2027, high-risk devices (class 3) need to be marked with a UDI and by end of 2028 devices with low and medium risk (class 1 & 2) also need to comply.
UDI – The facts
UDI stands for “Unique Device Identification” and is used to mark and identify devices within the healthcare supply chain. With this in mind, the US Food and Drug Administration and European Union have respectively passed new legislation. This affects the entire range of medical devices from
Class 1 (low risk, such as bandages etc.) to
Class 2 (medium risk, such as hearing aids, catheters) through to
Class 3 (high-risk devices for life-sustaining measures, such as pacemakers).
The UDI rule requires that the medical device as well as all higher packaging levels (not logistics units) are marked with a unique UDI code in a machine-readable form (barcode) and human readable plain text. Direct marking on the product itself is required for such medical devices, which are designed for repeated use over a long period of time and need to be prepared repeatedly, as these inevitably become separated from the original packaging. Various technologies (thermal ink jet, thermal transfer or laser) are used to apply the UDI, depending on the material in question.
Objectives of the UDI rule
- To establish a system to appropriately identify medical devices throughout the supply chain and during their use.
- Access to important and comprehensive information regarding the medical device.
- Opportunity to have standardized documentation on using medical devices in electronic patient files.
Each administration has created a database for this purpose. In the USA, this is the GUDID and in the European Union it is the EUDAMED database. The manufacturer is now required to register each product with its corresponding data elements in the administration database.
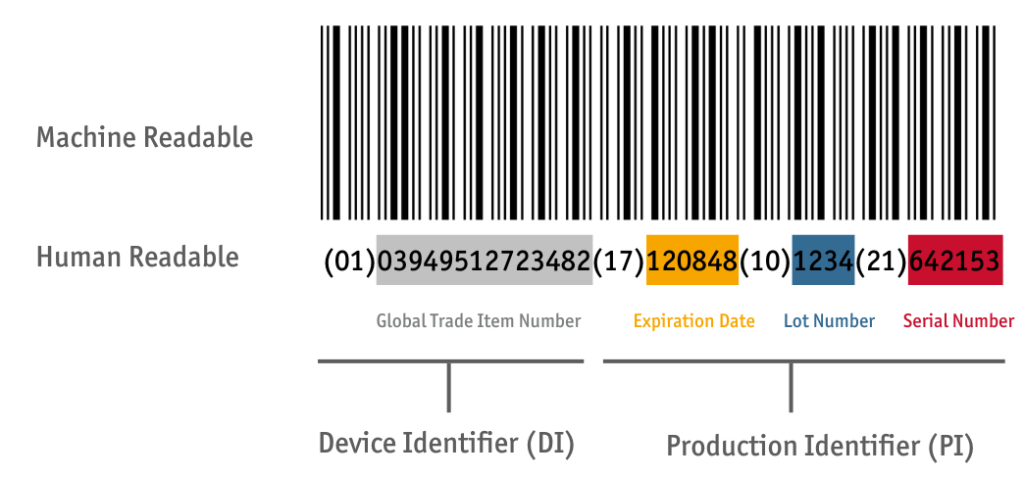
Structure of the UDI code
The terms and acronyms associated with the UDI rule may easily cause confusion. Here are some definitions to guide you:
UDI = “Unique Device Identification” – the coding on the product; corresponds to the GS1 Standard. UDI comprises DI + PI
DI = “Device Identifier” – Global Trade Item Number (GTIN)
PI = “Production Identifier” – Further production-related identifiers such as
- Lot: AI(10),
- expiration date: AI(17) or
- serial number: AI(21).
It is at the manufacturer’s discretion as to which information is used.
=> The UDI coding therefore comprises the GTIN and optional production-relevant information.
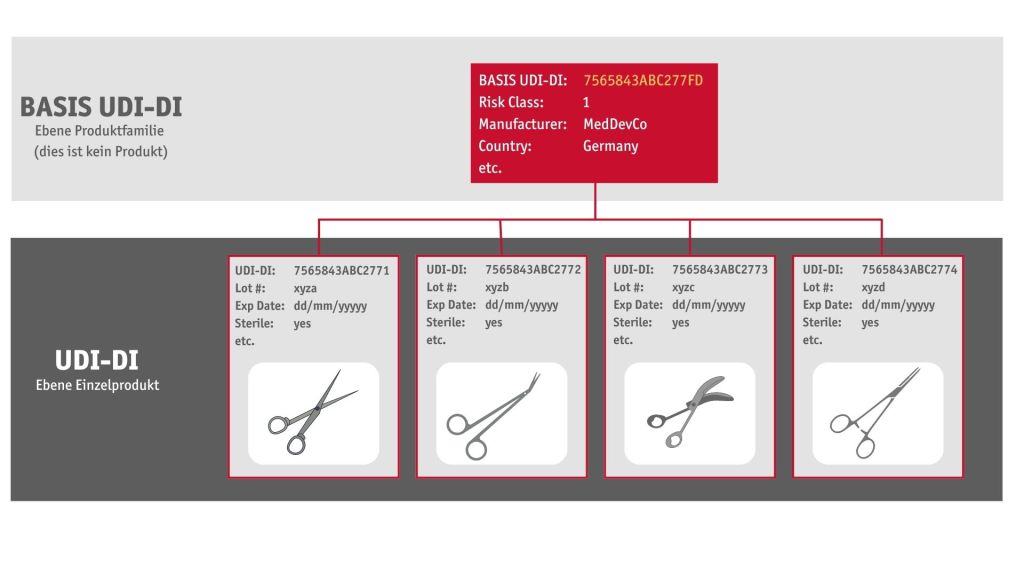
In contrast to the USA, an additional “Basic UDI-DI” is required for Europe. The “Basis UDI-DI” marks an entire product range. In addition to the same “Basis UDI-DI”, each individual product from this product range has its own unique number. This number is the UDI-DI. The “Basis UDI-DI” appears in the EUDAMED database and official documents. It never appears on the product itself or its packaging.
The UDI-DI (GTIN) appears in the EUDAMED database and on the products and/or their packaging. The product is then identified in the administration database based on the UDI-DI (GTIN) and all product information is made available to the person requesting it.
Regulations: Medical devices vs. Pharmaceutical
Currently, Track & Trace is only required for pharmaceutical products. Through deploying and using an identification system, companies which produce pharmaceuticals and medical products, become carriers of the information which is required for Track & Trace. This information can be used to identify each individual item. Initiatives are already in place, which recommend handling medical devices in exactly the same way as pharmaceutical products. We believe that in the near future manufacturers of medical devices will adopt the pharmaceutical model and will need to automate their product tracking information.
With this in mind, we recommend that all manufacturers of medical devices start using a Laetus Track & Trace system, even before any direct serialization requirements exist. Laetus has decades of experience in the pharmaceutical industry and with its large pharmaceutical customer base, is the ideal partner to accompany you taking your first steps in the field of Track & Trace.
Nevertheless, a Track & Trace system from Laetus can offer added value in many respects for manufacturers of medical devices, even without serialization.
In particular for manufacturers of large volumes, with numerous production lines at several locations, it makes sense to use a higher level system, which automatically distributes orders to the production lines and provides the respective printers and cameras with the correct data. Errors caused by operators loading an incorrect layout are eliminated. The system is able to directly communicate with your ERP to receive, process and report back on order data. Furthermore, uniform protocols and change records increase production quality.
Why serialization is the practical choice
Serialization and aggregation make a product traceable throughout the entire supply chain. Particularly when throughput is high, it is essential to know how many products have been produced and where they are in the supply chain. Consequently, traceability of products also offers tremendous internal added value.
Preferred is a system which can be used for both UDI regulations and pharmaceutical regulations, whilst providing the highest level of flexibility. It is therefore advisable to design a system to support serialization, which also includes UDI requirements and vice versa. If a requirement is issued in future to automate the exchange of information, the serialization structure created for pharmaceuticals can also be used for medical products. We recommend a holistic approach when it comes to developing your UDI system, since the safety of the supply chain and potential counterfeits will be a constant matter to contend with.
Conclusion
In summary, organisations should develop their UDI implementation strategy while taking the overall picture into consideration, and design solutions, which meet current requirements, but also take potential Track & Track requirements into account. Setting up a flexible solution may call for more substantial investment in the short term, but does however provide considerable savings in the long term.
