
Serialization as a Service (SaaS+)
How serialization can be transferred to the pharmaceutical supply chain
MSK Pharmalogistic is a family-run pharmaceutical service provider. Its core competence is in the field of pharmaceutical logistics, packaging and contract packaging. Since the company was founded over twenty years ago: MSK has continued to develop from being a sole contract packager and logistics expert to a full service provider for the pharmaceutical industry. The company’s range of services now extends from logistics to packaging through to complete fulfilment including sales and personnel solutions.
Since the delegated regulation of the 2011/62/EU directive known as the Falsified Medicines Directive (FMD) was published, one thing has become clear: The pharmaceutical industry has a duty to mark the packaging of its prescriptive pharmaceuticals with unique serial numbers and provide a tamper-evident safety seal. Anyone who fails to do so by 9th February 2019, will no longer be able to place their pharmaceuticals on the European market.
As a partner of the pharmaceutical industry, MSK wants to efficiently support its customers in overcoming this challenge. With this in mind, MSK was looking for solutions to be able to add the implementation of serialisation requirements to its range of services.
Back-up or complete transfer – Customers have the choice with SaaS+
MSK joined forces with Laetus and tracekey, an IT provider for the pharmaceutical industry. They have worked together to develop a concept to serialize products from customers following packaging in a GMP-certified production environment: Serialization as a Service (SaaS+) enables customers to completely transfer compliance requirements from production to the supply chain, if required. Alternatively, the service can be used as a back-up for production peaks, unplanned production bottlenecks or emergencies.
GDP-certified (Good Distribution Practice) transport to a 4,500 m2 temperature-controlled warehouse and quarantined storage are included in their range of services. Packaging, serialisation and subsequent optical control as well as complete data management including reporting to the supervisory authorities are then performed. Reintegration into the customer’s supply chain or complete supply chain logistics then takes place.
The technical expertise regarding serialisation and hardware and software equipment come from Laetus. The MV-50 forms the machine basis for SaaS+, a compact pack handling system, whose open construction enables a wide range of different marking systems to be used. As the system offers two print positions, folding boxes can not only be precisely marked on the front but also on the rear side to a high quality. In connection with powerful S-TTS (Secure Track & Trace Solutions) serialisation software, the serialisation is subsequently verified and quality is approved based on ISO/IEC standards. Depending on the general conditions, up to 400 folding boxes can be reliably serialised with the system per minute.
An efficient alternative for secure serialization
SaaS+ offers customers a secure and predominantly efficient solution to implement EU FMD regulations on time in a flexible manner. The so far unique service offers increased flexibility, security and cost-effectiveness for packaging processes and the supply chain.
Keys to success:
- Simply outsource EU-FMD compliance
- Flexible service based on current demands
- Proven Laetus technology ensures secure serialisation
- SaaS+ – concentrated serialisation expertise
Laetus products used
-
Laetus S-TTS
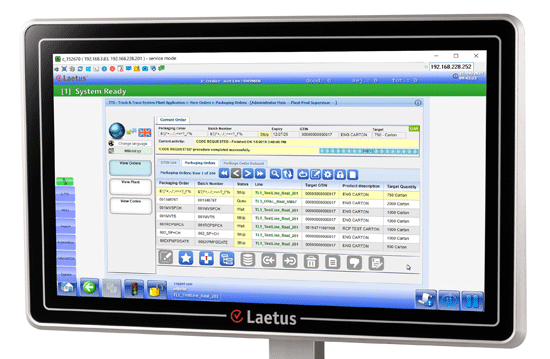
Modular secure track & trace software for seamless product tracking.
-
MV-50
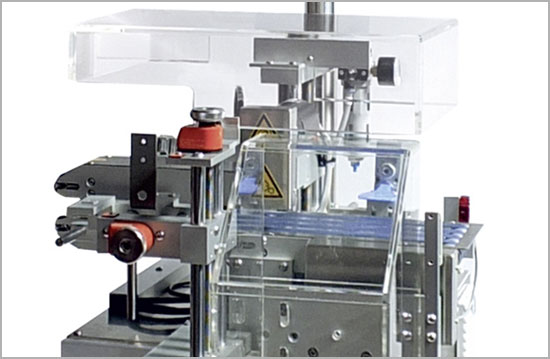
Hardware for flexible marking and verifying of serialized cartons.