
Setting up Serialization for China and South Korea
Fast Integration and Acceptance Reduce Downtime
EVER Pharma Jena GmbH in Germany produces and assembles sterile liquid preparations in ampules, vials, and ready-to-fill syringes. Medicinal products for the major sales markets of China and South Korea are packaged on a high-performance line, which processes some 60 percent of the company’s products. That was why it was particularly important that the downtime of the line was kept to a minimum during the implementation and qualification phases of the serialization solution. One further challenge was the large space required by code 128, a linear barcode, required by the regulations of the China Food and Drug Administration (CFDA).
These requirements have been successfully implemented with three machines which are integrated into the existing line configuration and an independent, manual pack handling system for the warehouse. Right after the checkweigher, which checks if the folding box is complete, follows a Pack Handling System MV-50, which prints the codes onto the folding boxes and then verifies them. In another packaging process, a further Pack Handling System, the IMV-370, performs the aggregation of bundles of data as required by Korea and affixes the corresponding label to the bundle. Then, using a manual CS-60 hand packaging station, the serial numbers of the bundle are scanned in and linked to a shipping aggregation. A further CS-60 is put to use for the subsequent processing steps.
The data handling is carried out by the Secure Track & Trace Software (S-TTS) from Laetus, which processes all serial and production-related data and is responsible for the management and organization of the Track & Trace process. The solution can be entirely removed during the FAT, which kept downtimes to a minimum.
Solution Approaches & Facts
- Efficient pre-project analysis and planning
- Rapid machine integration and system qualification
Summary/Statements
Edo Kuntzsch, Head of Process Engineer at EVER Pharma, said:
“We are very pleased with the project’s progress and the result. The decisive criterion for choosing a supplier was, among other things, respect for the tight overall project timeline, plus speedy machine integration and system qualification during the implementation phase. Due to a close cooperation with Laetus, we were able to achieve this successfully.“
A thorough pre-project planning phase, actioned by the project team responsible, made it possible not to increase the space required by the overall line, despite adding machines.
Powered by Laetus products:
-
Laetus S-TTS
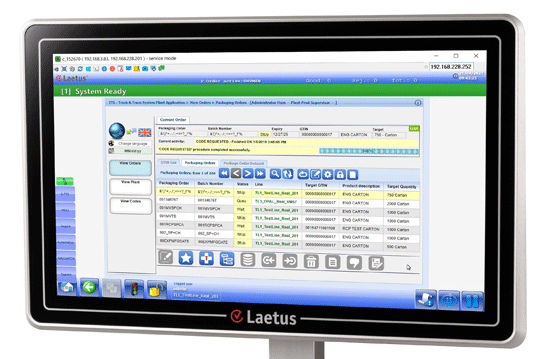
Modular secure track & trace software for seamless product tracking.
-
MV-50
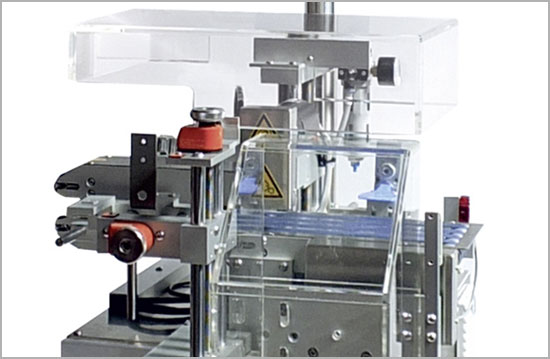
Hardware for flexible marking and verifying of serialized cartons.